Table of Contents
ToggleDefine Electrolysis
Electrolysis is the passage of a direct electric current through an ionic substance that is either molten or dissolved in a suitable solvent, resulting in chemical reactions at the electrodes and separation of materials. The main components required to achieve electrolysis are :
Electrolyte : a substance containing free ions which are the carriers of electric current in the electrolyte. If the ions are not mobile, as in a solid salt then electrolysis cannot occur.
Direct current (DC) supply : provides the energy necessary to create or discharge the ions in the electrolyte. Electric current is carried by electrons in the external circuit.
Two electrodes : an electrical conductor which provides the physical interface between the electrical circuit providing the energy and the electrolyte Electrodes of metal, graphite and semiconductor material are widely used. Choice of suitable electrode depends on chemical reactivity between the electrode and electrolyte and the cost of manufacture.
Process of electrolysis
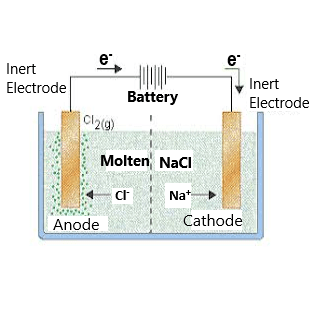
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons from the external circuit. The required products of electrolysis are in some different physical state from the electrolyte and can be removed by some physical processes. For example, in the electrolysis of brine to produce hydrogen and chlorine, the products are gaseous. These gaseous products bubble from the electrolyte and are collected.
2 NaCl + 2 H2O → 2 NaOH + H2 + Cl2
A liquid containing mobile ions (electrolyte) is produced by
- Solvation or reaction of an ionic compound with a solvent (such as water) to produce mobile ions
- An ionic compound is melted (fused) by heating
An electrical potential is applied across a pair of electrodes immersed in the electrolyte. Each electrode attracts ions that are of the opposite charge. Positively charged ions (cations) move towards the electron-providing (negative) cathode, whereas negatively charged ions (anions) move towards the positive anode.
At the electrodes, electrons are absorbed or released by the atoms and ions. Those atoms that gain or lose electrons to become charged ions pass into the electrolyte. Those ions that gain or lose electrons to become uncharged atoms separate from the electrolyte. The formation of uncharged atoms from ions is called discharging. The energy required to cause the ions to migrate to the electrodes, and the energy to cause the change in ionic state, is provided by the external source of electrical potential.
Energy changes during electrolysis
The amount of electrical energy that must be added equals the change in Gibbs free energy of the reaction plus the losses in the system. The losses can (in theory) be arbitrarily close to zero, so the maximum thermodynamic efficiency equals the enthalpy change divided by the free energy change of the reaction. In most cases, the electric input is larger than the enthalpy change of the reaction, so some energy is released in the form of heat. In some cases, for instance, in the electrolysis of steam into hydrogen and oxygen at high temperature, the opposite is true. Heat is absorbed from the surroundings, and the heating value of the produced hydrogen is higher than the electric input.
Industrial Application of Electrolysis
Hall-Heroult process for producing aluminium
1.Production of aluminium, lithium, sodium, potassium, magnesium, calcium
2.Coulometric techniques can be used to determine the amount of matter transformed during electrolysis by measuring the amount of electricity required to perform the electrolysis
3.Production of chlorine and sodium hydroxide
4.Production of sodium chlorate and potassium chlorate
5.Production of perfluorinated organic compounds such as trifluoroacetic acid
6.Production of electrolytic copper as a cathode, from refined copper of lower purity as an anode.
Electrolysis has many other uses:
1.Electrometallurgy is the process of reduction of metals from metallic compounds to obtain the pure form of metal using electrolysis. For example, sodium hydroxide in its molten form is separated by electrolysis into sodium and oxygen, both of which have important chemical uses. (Water is produced at the same time.)
2.Anodization is an electrolytic process that makes the surface of metals resistant to corrosion. For example, ships are saved from being corroded by oxygen in the water by this process. The process is also used to decorate surfaces.
3.A battery works by the reverse process to electrolysis.
4.Production of oxygen for spacecraft and nuclear submarines.
5.Electroplating is used in layering metals to fortify them. Electroplating is used in many industries for functional or decorative purposes, as in vehicle bodies and nickel coins.
6.Production of hydrogen for fuel, using a cheap source of electrical energy.
7.Electrolytic Etching of metal surfaces like tools or knives with a permanent mark or logo.

Excellent read, I just passed this onto a friend who was doing some research on that. And he just bought me lunch as I found it for him smile Therefore let me rephrase that: Thanks for lunch!
This is the right blog for anyone who needs to seek out out about this topic. You realize a lot its virtually hard to argue with you (not that I truly would need…HaHa). You undoubtedly put a brand new spin on a topic thats been written about for years. Great stuff, simply nice!